William Holmes Research Page
Documents of Interest:
Background:
I received my B.S. in Engineering Physics form the University of Tennessee at Knoxville in 2005. As an undergraduate I participated in a Co-Op program that allowed me to work at Rolls Royce Aerospace (Indianapolis, IN) for two terms. During the first I developed software to perform various tasks of interest to the Materials Review Board. During the second, I worked in the Experimental Test Department designing and implementing testing methods for aircraft engine components, primarily the vertical lift fan for the joint strike fighter.
After completing my B.S., I went on to pursue a PhD (2005-2010) in mathematics, with a minor in scientific computing, at Indiana University Bloomington. While there, my primary PhD work consisted of developing and studying a 3D mechanistic model of the mammalian cochlea. Upon completing my PhD, I took a postdoc (2010 - 2012) in the Mathematics Department at the University of British Columbia working with Professor Leah Edelstein-Keshet. There I moved more toward cell biology and began working to understand spatiotemporal regulation of activities such as chemotaxis and cytokinesis.
I am now an assistant researcher (2012 - present) in the Mathematics Department at the University of California at Irvine where I continue to work in the cellular systems biology realm along with more recent interests in Bayesian modeling of cognative phenomena.
Research Interests:
Broadly I am interested in the application of mathematical methods to describe and study problems stemming from life and social sciences. My PhD work involved the application of PDE, asymptotics, and numerical analysis to the study human hearing (Cochlear function to be more specific). These days I work at the interface of cellular systems biology and mathematics. Broadly, I work to detail and understand the mechanisms responsible for highly choreographed spatio temporal regulation of cellular processes such as chemotaxis (movement of a cell in response to a chemical gradient), cytokinesis (cell division), wound regeneration, and embryonic development. In mathematical terms, this boils down to constructing descriptive models (typically ODE, PDE, or discrete stochastic models) of biological processes, studying their properties, and connecting results of those investigations to the motivating systems to suggest targeted experiments and hopefully provide new insights.
The use of mathematics to investigate biological systems is the prominent theme of this program. But these investigations have led to the development of new mathematical techniques as well. A substantial focus of my work is developing high throughput, user friendly methods (the Local Perturbation Analysis) for detecting and classifying patterning in complex, spatial regulatory networks.
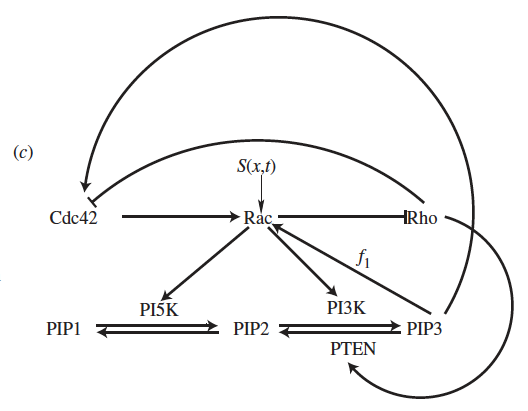
Schematic of a polarity network.
Finite element simulation of a propogating actin wave.
Symmetry breaking and polarization in chemotaxis
A number of events must occur in order for a cell to move in response to a chemotactic gradient. First, the external signal must be internalized via transmembrane receptors. Second, that signal must be interpreted. Third, remodelling of the biophysical elements that give a cell its structure (actin, myosin, etc.) must occur. The second step, of primary interest to me, is known to be mediated by a collection proteins (Rho GTPases) and lipids (phosphoinositides). The applied stimulus (which can vary by as little as 2-3% over the length of a cell in some cases) causes these molecules to reorganize creating segregated regions where some move primarily to the "front" and others to the "back" of the cell. This choreographed molecular reorganization then regulates growth and contraction of the cells cytoskeleton leading to directed motion.
The primary question of interest is simply how do these molecules self-organize in response to a stimulus. Do the biochemical properties of the molecules themselves allow them to reorganize robustly or are the specific network interactions responsible? How does an applied stimulus interact with these regulatory networks? How sensitive are these networks and what might control that sensitivity? These systems can be reposed mathematically in the language of reaction diffusion systems and such questions can be discussed in the context of pattern formation and symmetry breaking which has a rich history.
In this view of motility, the three events are sequential with one following from the other. However the presence of highly dynamic activity of the cytoskeleton such as actin waves, spots, boundary protrusion waves, and breathers (rhythmic uniform expansion and contraction of the entire cell) suggest that some sort of feedback between cytoskeletal remodelling and its regulation is present. This raises questions such as how does the cytoskeleton itself influence its regulators? How does this effect the regulatory biochemistry and lead to dynamic behavior? What biological role would such feedbacks have?
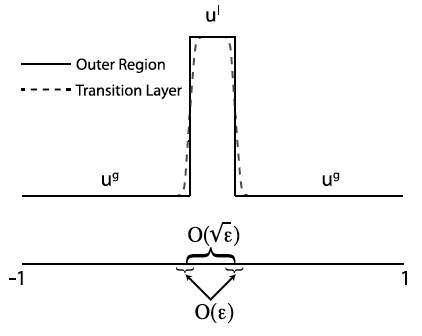
Schematic of a local pulse perturbation.
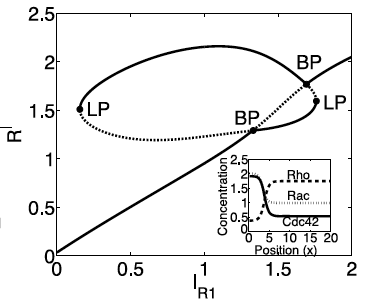
LPA bifircation diagram for a polarity network.
The Local Perturbation Analysis
Many of the questions mentioned above require describing complex biological interaction networks in terms of reaction diffusion equations and studying their properties. Substantial work has been done over the past 70-100 years on detecting and describing pattern formation in reaction diffusion systems. These methods primarily fall into two categories, linear and nonlinear. Linear methods (ie. well mixed and Turing analyses) describe the growth or decay of a vary small perturbation of a known homogeneous steady state. These methods are limited in that they can only detect instabilities to small perturbations. Nonlinear methods (ie. weakly nonlinear analysis, asymptotic methods, full PDE bifurcation analysis) provide substantially more information, but tend to be very difficult to apply and are rarely applicable to systems of more than two or three variables.
Biochemical systems tend to be quite complicated, ruling out the use of nonlinear methods. Typical linear methods can be applied to complex systems, but still require the expertise to solve large eigenvalue problems. Furthermore, it is often of interest to discuss the sensitivity of a biochemical network. This requires consideration of large amplitude perturbation for which linear methods are unsuitable. To bridge this gap, I (along with Stan Maree and Veronica Grieneisen at the John Innes Centre) have been developing a new type of stability analysis referred as the Local Perturbation Analysis that is applicable to a large class of biological systems involving regulators that are either bound to a membrane or freely diffusing. This method has proven immensely useful in practical applications but still requires substantial work to fully characterize its scope of application and the type of information that can be reliably obtained with it.
For those interested in using this method, I have compiled some tutorial style slides and examples (with code) as part of a previous short course. These materials can be found here.
Cytokinesis
Cytokinesis is a complicated process, but at a basic level three processes are required. 1) Specification of a division plane, indicated by a ring of membrane activity enriched with certain necessary proteins. 2) Constriction of that ring. 3) Closure or cleavage of the constricted ring which completes division. Recent experimental observations have drawn my interest to the first of these processes. An intersting question is how enrichment of certain proteins (Rho for example) occurs. Taking one step back, a different interpretation of this question is what kind of spatial signal initiates this enrichment, and how does it do so. I have been recently working on each of these two questions in parallel.
First, what kind of signal is delivered to the membrane and how is it delivered there. Evidence in multiple cell types suggest microtubule motor associated proteins are responsible for carrying signalling cargo to the future cytokinetic ring. Beautiful imagry indicates that these stabalized microtubules only contact the membrane at the future site of this ring. But why is this the case. There is a rich literature suggesting that kinesin motors can modulate dynamic instability by promoting microtubule catastrophe (shortening). However, recent evidence suggests cytokinesis associated motors might do just the opposite, stabalize microtubulus. I have been doing both analytical and spatial stochastic simulations to understand how this process promotes microtubule and hence signalling localization.
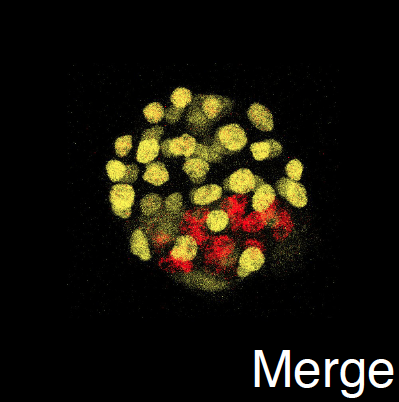
Flourescence image of an early stage embryo.
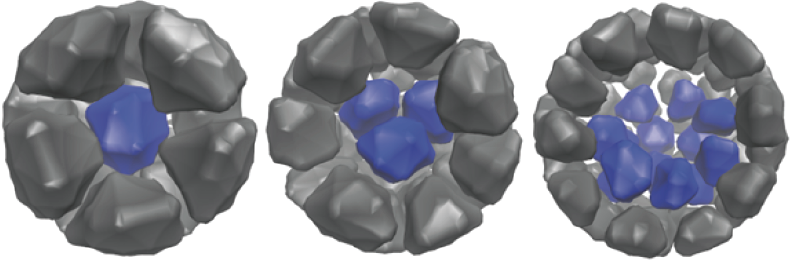
Discrete stochastic simulation of a developing embryo.
Embryonic Development
Emrbyonic development is an exquisitly complex yet highly robust process. This robustness is highlighed by the fact that an early developing embryo can actually break into two, leading to the development of identical twins. Even more surprising, the cells of an 8 cell embryo can be completely disassociated, with each giving rise to a normal embryo. What are the design principles responsible for development of the early mammalian embryo and how is it they can resist such extreme perturbations?
The first critical stage of in mammalian develeopment is the separation of an initial blob of ~16 cells into two distinct aggregates: and inner cell mass (ICM) surrounded by a shell of trophectoderm (TE) cells. This inner mass is what we typically think of as the embryo and it will give rise to all future embryonic structures. The TE on the other hand will give rise to all extra embryonic structures, e.g. the placenta. In collaboration with the Cho lab in Dev. and Cell Biology at UC Irvine, I have investigated the events that direct this early organizational event. Much of this work involves understanding how this organization can be maintained in the face of constant assault of stochasticity. Surprising initial results here suggest that contrary to popular belief, stochasticity is an organizing rather than a dis-organizing force.
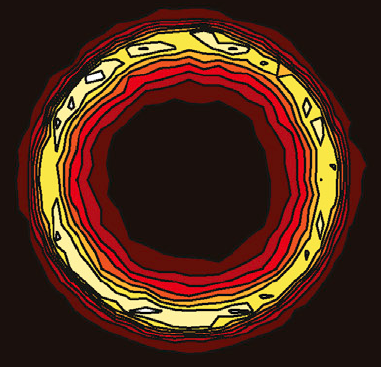
Simulation of a persistent CNS lesion.
Central Nervous System Regeneration
Given the prevalence and cost of chronic wounds, considerable efforts have been directed at understanding the choreographed events set in motion by damage that lead to repair and regeneration. Although wounds of the epidermis are possibly the best studied, lesions of the central nervous system (CNS) are arguably the most devastating and irreparable.
Common causes include damage from external sources and neurological disorders such as multiple sclerosis (MS), which affects >2 million worldwide and 300,000 in the United States (according to National Institute of Neurological Disorders and Stroke (NINDS)).
Regeneration of central nervous system (CNS) lesions requires movement of progenitor cells, proliferation and differentiatedof their progeny. But these events are not separable and can in fact interfere with each other. The goal of this work is to understand how these different processes can be chorographed to elicit a good regeneration response. Initial investigation has led to suggestions for how the endogenous response might be augmented to improve regeneration. Further work will expand this preliminary data to include important complexities not accounted for and gain a better understanding of how lesions form, what determines their size, and what can improve the endogenous regeneration response or make the lesion environment more permissable to stem cell transplant therapies.
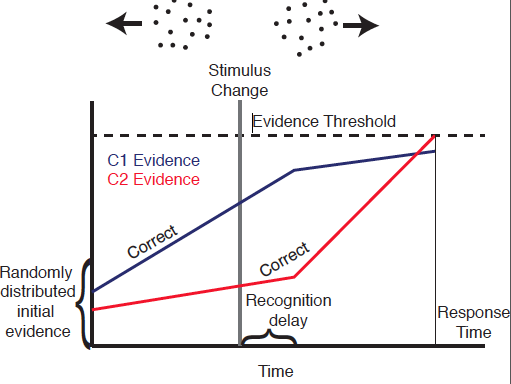
Schematic of a evidence accumulation model.
Bayesian Methods for Model Based Inference with Cognitive Behavioural Data
A critical task in modelling is to determine how well the theoretical assumptions encoded in a model account for observations. Bayesian methods are an ideal framework for doing just this. Markov Chain Monte Carlo, a widely used class Bayesian methods, is very powerful, but only in limited circumstances where a problem can be "analytically" described in terms of a likelihood function. In cases where this is not possible, numerous approximation methods have been developed. These methods however usually suffer from an inconsistency (in mathematical language) or insufficiency (in statistics language).
As part of a new collaboration, I am working to develop new approximate Bayesian techniques that overcome these difficulties. In particular, I am working to integrate non-parameteric statistical methods into the MCMC framework, incorporate high performance computing techniques to accelerate this method, and make it accessible to a broader audience. The scientific goals of this collaboration are to determine how dynamically changing information is integrated in the decision process in different contexts (e.g. perceputal decision making versus consumer choice or risky versus riskless contexts).